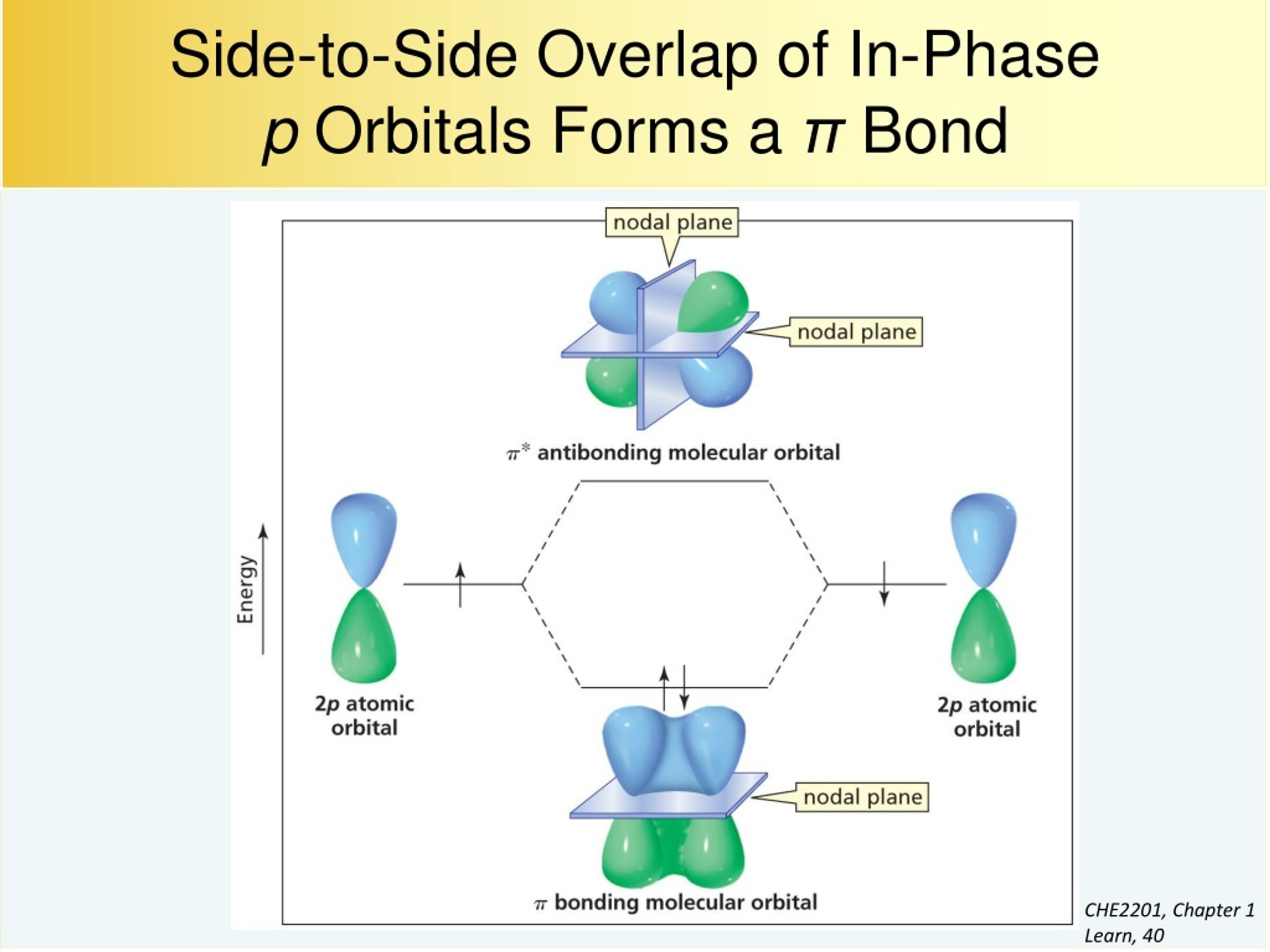
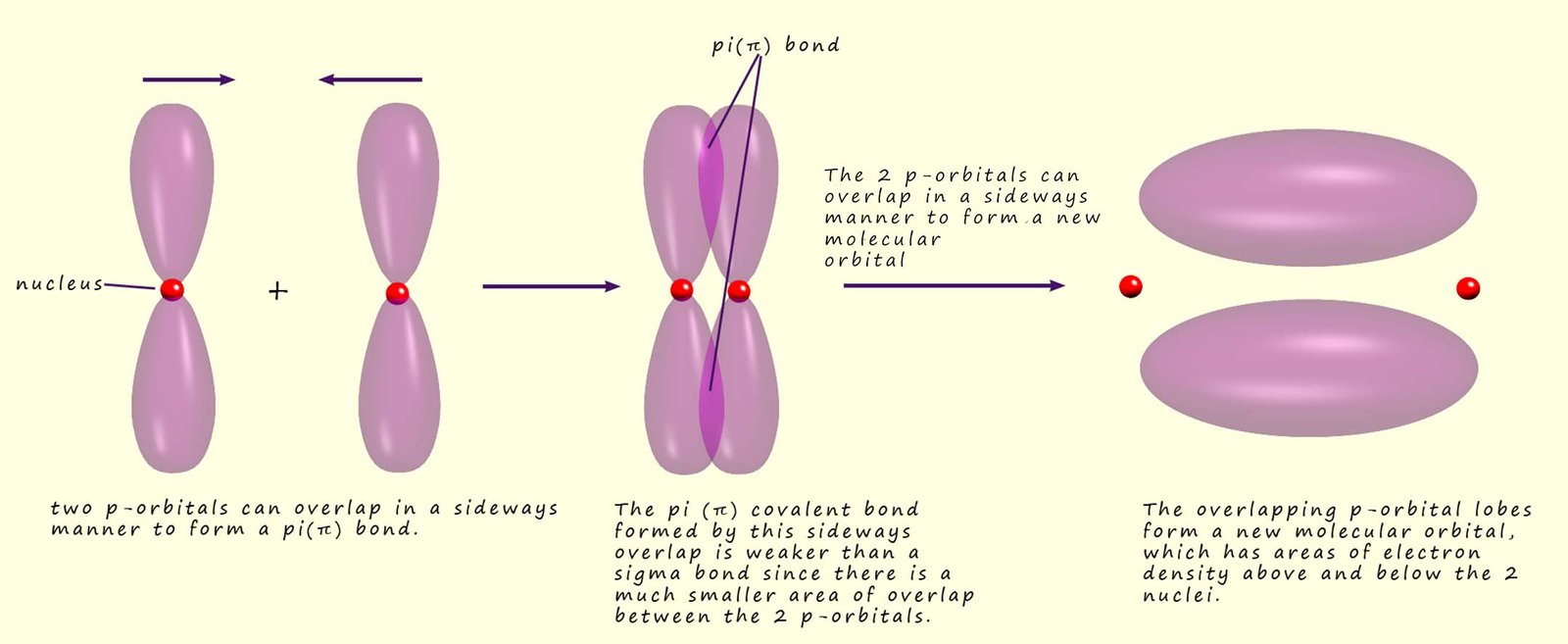
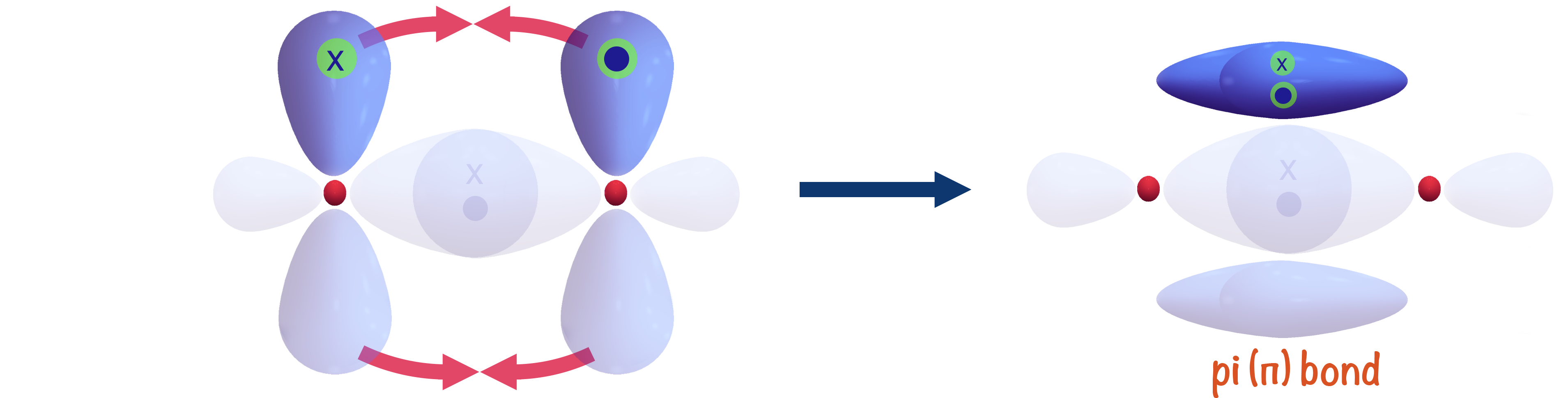
Which Orbitals Can Overlap To Form A Pi Bond - The orbitals in an atom are organized into different layers or electron shells. There are four types of orbitals that you should know (sharp, principal, diffuse and fundamental) with s, p, d and f. What are the 4 atomic orbitals? Orbitals in physics and chemistry is a mathematical function depicting the wave nature of an electron or a pair of electrons present. The difference between orbit and orbitals is important to understand for any budding chemistry student or even professionals in the field of. Recommended videos shapes of orbitals what is an orbital? The molecular orbital theory (often abbreviated to mot) is a theory on chemical bonding developed at the beginning. What is molecular orbital theory?
Recommended videos shapes of orbitals what is an orbital? Orbitals in physics and chemistry is a mathematical function depicting the wave nature of an electron or a pair of electrons present. The difference between orbit and orbitals is important to understand for any budding chemistry student or even professionals in the field of. What is molecular orbital theory? The molecular orbital theory (often abbreviated to mot) is a theory on chemical bonding developed at the beginning. The orbitals in an atom are organized into different layers or electron shells. There are four types of orbitals that you should know (sharp, principal, diffuse and fundamental) with s, p, d and f. What are the 4 atomic orbitals?
There are four types of orbitals that you should know (sharp, principal, diffuse and fundamental) with s, p, d and f. The orbitals in an atom are organized into different layers or electron shells. Orbitals in physics and chemistry is a mathematical function depicting the wave nature of an electron or a pair of electrons present. What are the 4 atomic orbitals? What is molecular orbital theory? Recommended videos shapes of orbitals what is an orbital? The difference between orbit and orbitals is important to understand for any budding chemistry student or even professionals in the field of. The molecular orbital theory (often abbreviated to mot) is a theory on chemical bonding developed at the beginning.
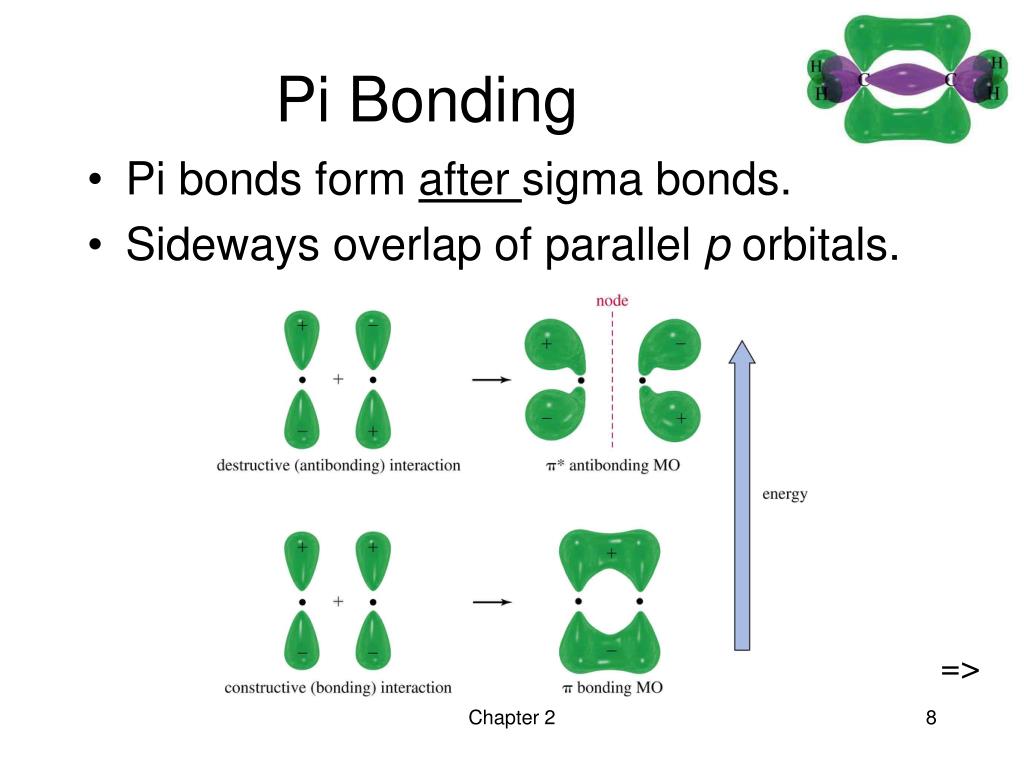
The overlap of two lobes of p orbitals in a pi bond. r/chemistry
The molecular orbital theory (often abbreviated to mot) is a theory on chemical bonding developed at the beginning. The difference between orbit and orbitals is important to understand for any budding chemistry student or even professionals in the field of. The orbitals in an atom are organized into different layers or electron shells. What are the 4 atomic orbitals? What.
PPT Chapter 2 Structure and Properties of Organic Molecules
The molecular orbital theory (often abbreviated to mot) is a theory on chemical bonding developed at the beginning. The orbitals in an atom are organized into different layers or electron shells. Recommended videos shapes of orbitals what is an orbital? Orbitals in physics and chemistry is a mathematical function depicting the wave nature of an electron or a pair of.
Chapters 9 and 11 study guide
Recommended videos shapes of orbitals what is an orbital? The molecular orbital theory (often abbreviated to mot) is a theory on chemical bonding developed at the beginning. There are four types of orbitals that you should know (sharp, principal, diffuse and fundamental) with s, p, d and f. The difference between orbit and orbitals is important to understand for any.
Chapter 7 Chemical Bonding. ppt download
Recommended videos shapes of orbitals what is an orbital? The orbitals in an atom are organized into different layers or electron shells. There are four types of orbitals that you should know (sharp, principal, diffuse and fundamental) with s, p, d and f. Orbitals in physics and chemistry is a mathematical function depicting the wave nature of an electron or.
Chemical Bonding and Molecular Structure Class 11 Notes CBSE Chemistry
There are four types of orbitals that you should know (sharp, principal, diffuse and fundamental) with s, p, d and f. Recommended videos shapes of orbitals what is an orbital? What are the 4 atomic orbitals? What is molecular orbital theory? Orbitals in physics and chemistry is a mathematical function depicting the wave nature of an electron or a pair.
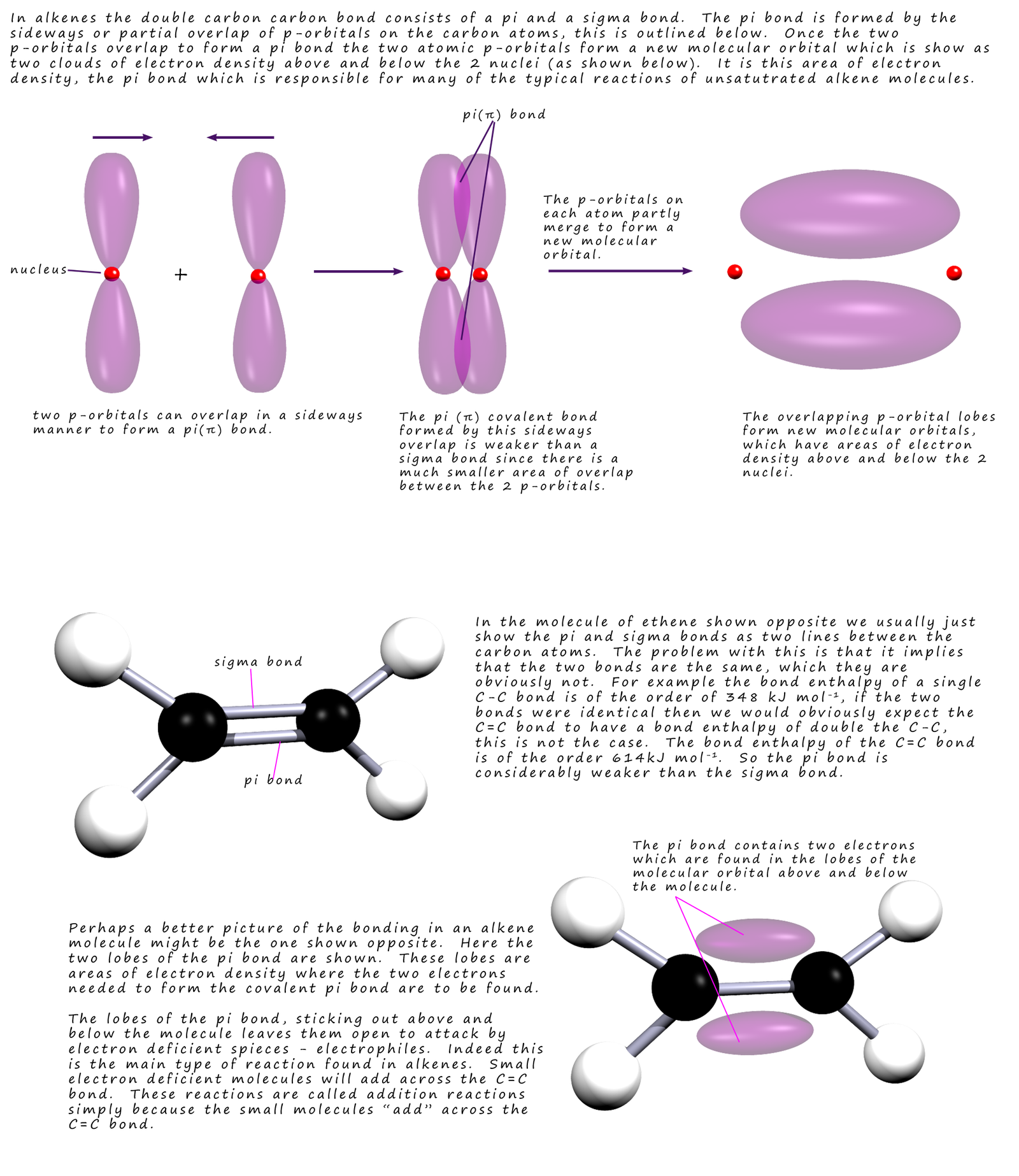
Electrophilic addition
Orbitals in physics and chemistry is a mathematical function depicting the wave nature of an electron or a pair of electrons present. Recommended videos shapes of orbitals what is an orbital? What are the 4 atomic orbitals? What is molecular orbital theory? There are four types of orbitals that you should know (sharp, principal, diffuse and fundamental) with s, p,.
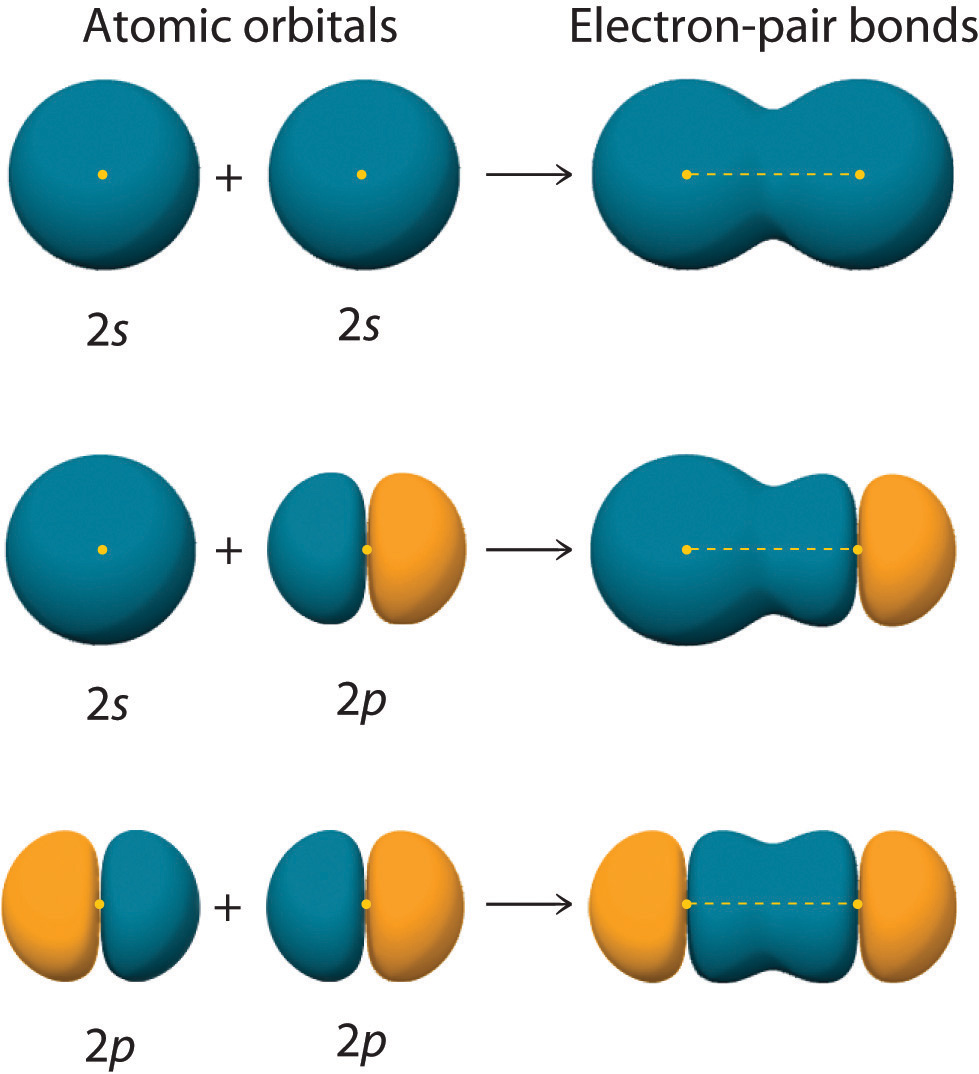
9.4 Covalent Bonding and Orbital Overlap Chemistry LibreTexts
There are four types of orbitals that you should know (sharp, principal, diffuse and fundamental) with s, p, d and f. The molecular orbital theory (often abbreviated to mot) is a theory on chemical bonding developed at the beginning. The difference between orbit and orbitals is important to understand for any budding chemistry student or even professionals in the field.
PPT Structure and Bonding PowerPoint Presentation, free download ID
The orbitals in an atom are organized into different layers or electron shells. There are four types of orbitals that you should know (sharp, principal, diffuse and fundamental) with s, p, d and f. What is molecular orbital theory? Recommended videos shapes of orbitals what is an orbital? The difference between orbit and orbitals is important to understand for any.
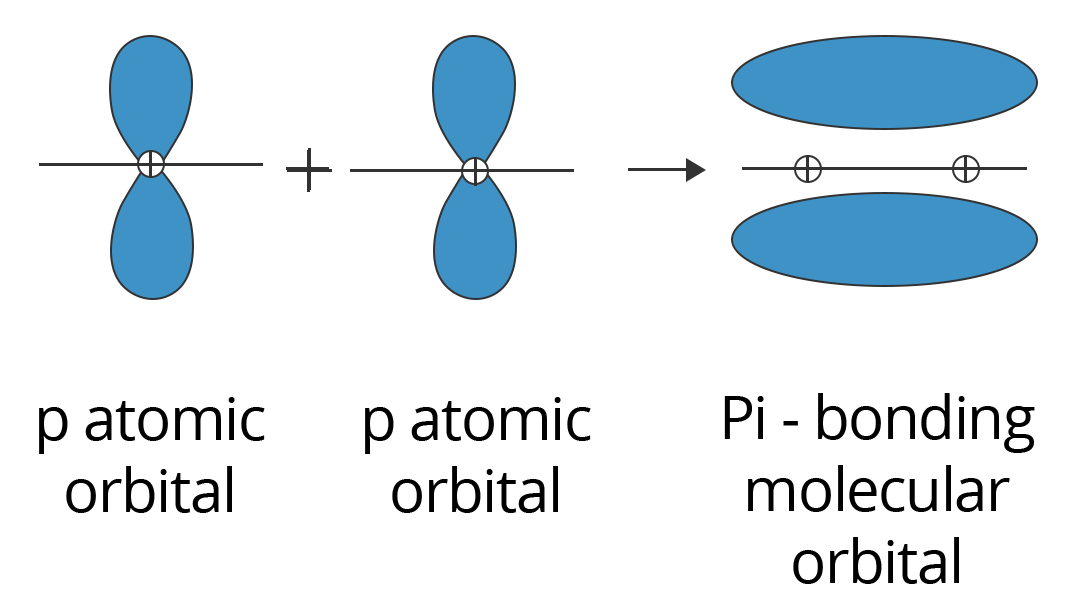
Sigma and pi bonds
What is molecular orbital theory? The difference between orbit and orbitals is important to understand for any budding chemistry student or even professionals in the field of. What are the 4 atomic orbitals? There are four types of orbitals that you should know (sharp, principal, diffuse and fundamental) with s, p, d and f. Recommended videos shapes of orbitals what.
Sigma (δ) and Pi ((π) Bond (ALevel) ChemistryStudent
The orbitals in an atom are organized into different layers or electron shells. Recommended videos shapes of orbitals what is an orbital? The molecular orbital theory (often abbreviated to mot) is a theory on chemical bonding developed at the beginning. Orbitals in physics and chemistry is a mathematical function depicting the wave nature of an electron or a pair of.
The Molecular Orbital Theory (Often Abbreviated To Mot) Is A Theory On Chemical Bonding Developed At The Beginning.
Recommended videos shapes of orbitals what is an orbital? The difference between orbit and orbitals is important to understand for any budding chemistry student or even professionals in the field of. There are four types of orbitals that you should know (sharp, principal, diffuse and fundamental) with s, p, d and f. Orbitals in physics and chemistry is a mathematical function depicting the wave nature of an electron or a pair of electrons present.
What Are The 4 Atomic Orbitals?
The orbitals in an atom are organized into different layers or electron shells. What is molecular orbital theory?