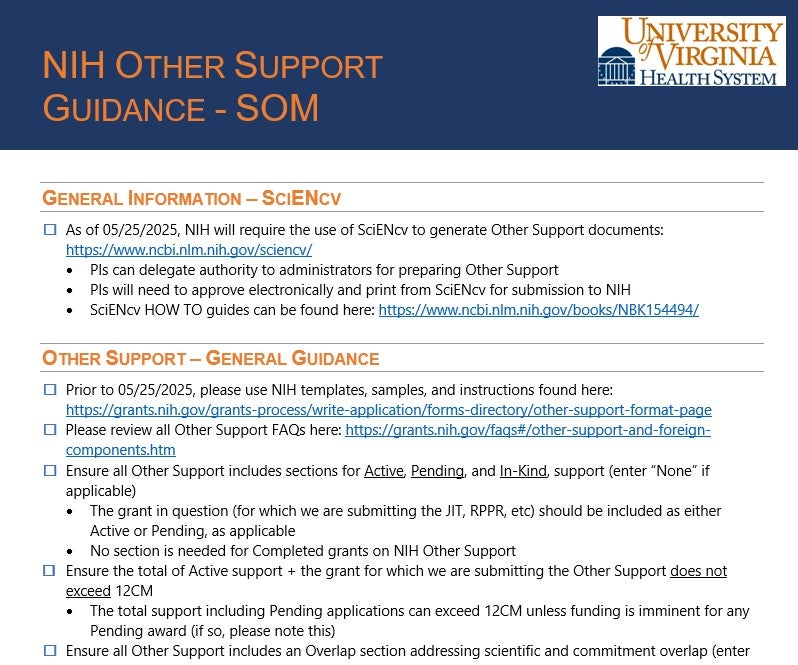
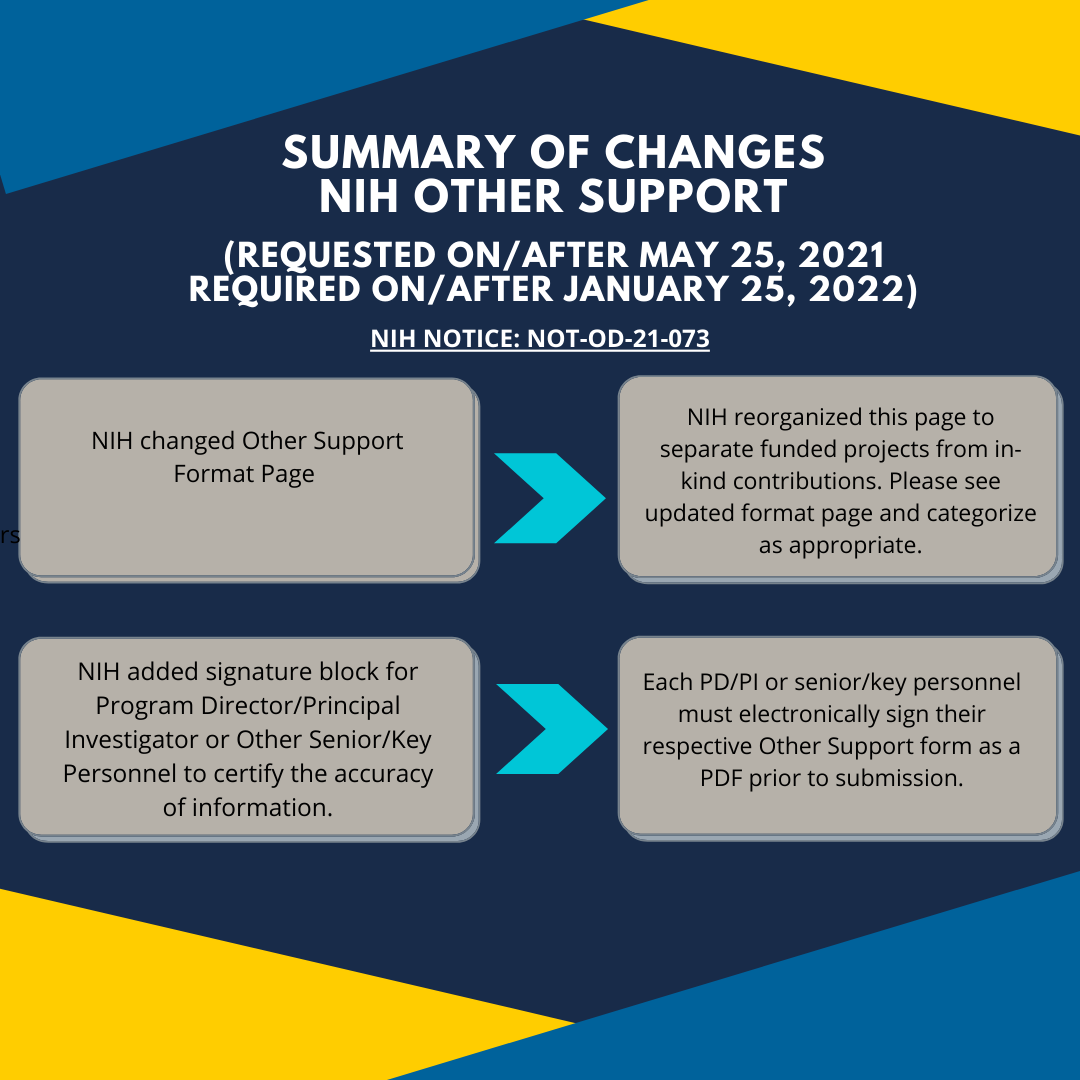
Nih Other Support Template 2025 - The nih's adoption of the common forms (biosketch and current and pending (other) support) originally scheduled for may 25, 2025 is. Nih's adoption of the common form for current and pending (other) support originally scheduled for may 25, 2025 is postponed. “other support” is sometimes referred to as “current and pending support” or “active and pending support.” find instructions, blank format pages,. The new requirement to train researchers to comply with other support disclosure requirements adds to the current policy (see nih. This guide notice informs the extramural community of nih’s adoption of common forms for biographical sketch and current and. Continue to use the nih. As the largest public funder of biomedical research in the world, nih supports a variety of programs from grants and contracts to. Applicants/recipients will be required to use science experts network curriculum vitae (sciencv) for preparing and certifying nih.
This guide notice informs the extramural community of nih’s adoption of common forms for biographical sketch and current and. Continue to use the nih. Nih's adoption of the common form for current and pending (other) support originally scheduled for may 25, 2025 is postponed. The new requirement to train researchers to comply with other support disclosure requirements adds to the current policy (see nih. “other support” is sometimes referred to as “current and pending support” or “active and pending support.” find instructions, blank format pages,. The nih's adoption of the common forms (biosketch and current and pending (other) support) originally scheduled for may 25, 2025 is. As the largest public funder of biomedical research in the world, nih supports a variety of programs from grants and contracts to. Applicants/recipients will be required to use science experts network curriculum vitae (sciencv) for preparing and certifying nih.
As the largest public funder of biomedical research in the world, nih supports a variety of programs from grants and contracts to. The nih's adoption of the common forms (biosketch and current and pending (other) support) originally scheduled for may 25, 2025 is. Nih's adoption of the common form for current and pending (other) support originally scheduled for may 25, 2025 is postponed. The new requirement to train researchers to comply with other support disclosure requirements adds to the current policy (see nih. This guide notice informs the extramural community of nih’s adoption of common forms for biographical sketch and current and. Applicants/recipients will be required to use science experts network curriculum vitae (sciencv) for preparing and certifying nih. Continue to use the nih. “other support” is sometimes referred to as “current and pending support” or “active and pending support.” find instructions, blank format pages,.
Other Support, Related Effort, and RMS Systems ppt download
The nih's adoption of the common forms (biosketch and current and pending (other) support) originally scheduled for may 25, 2025 is. As the largest public funder of biomedical research in the world, nih supports a variety of programs from grants and contracts to. Nih's adoption of the common form for current and pending (other) support originally scheduled for may 25,.
PPT National Institutes of Health “JustInTime” Procedures
Applicants/recipients will be required to use science experts network curriculum vitae (sciencv) for preparing and certifying nih. The new requirement to train researchers to comply with other support disclosure requirements adds to the current policy (see nih. As the largest public funder of biomedical research in the world, nih supports a variety of programs from grants and contracts to. The.
DSP Update USDA F&A & Match Equipment Rental Staffing Update ppt download
Applicants/recipients will be required to use science experts network curriculum vitae (sciencv) for preparing and certifying nih. The new requirement to train researchers to comply with other support disclosure requirements adds to the current policy (see nih. “other support” is sometimes referred to as “current and pending support” or “active and pending support.” find instructions, blank format pages,. As the.
Forms, Templates, & Resources Grants and Contracts
Nih's adoption of the common form for current and pending (other) support originally scheduled for may 25, 2025 is postponed. Continue to use the nih. This guide notice informs the extramural community of nih’s adoption of common forms for biographical sketch and current and. As the largest public funder of biomedical research in the world, nih supports a variety of.
New NIH Other Support keck usc Doc Template pdfFiller
The new requirement to train researchers to comply with other support disclosure requirements adds to the current policy (see nih. Continue to use the nih. The nih's adoption of the common forms (biosketch and current and pending (other) support) originally scheduled for may 25, 2025 is. “other support” is sometimes referred to as “current and pending support” or “active and.
PI Quick Guide re NIH Other Support and Biosketch Google Drive
“other support” is sometimes referred to as “current and pending support” or “active and pending support.” find instructions, blank format pages,. As the largest public funder of biomedical research in the world, nih supports a variety of programs from grants and contracts to. Nih's adoption of the common form for current and pending (other) support originally scheduled for may 25,.
Other Support Fill out & sign online DocHub
The nih's adoption of the common forms (biosketch and current and pending (other) support) originally scheduled for may 25, 2025 is. “other support” is sometimes referred to as “current and pending support” or “active and pending support.” find instructions, blank format pages,. Nih's adoption of the common form for current and pending (other) support originally scheduled for may 25, 2025.
Nih Facilities And Other Resources Template
Continue to use the nih. Nih's adoption of the common form for current and pending (other) support originally scheduled for may 25, 2025 is postponed. The new requirement to train researchers to comply with other support disclosure requirements adds to the current policy (see nih. As the largest public funder of biomedical research in the world, nih supports a variety.
Other Support sample Doc Template pdfFiller
Applicants/recipients will be required to use science experts network curriculum vitae (sciencv) for preparing and certifying nih. The nih's adoption of the common forms (biosketch and current and pending (other) support) originally scheduled for may 25, 2025 is. Nih's adoption of the common form for current and pending (other) support originally scheduled for may 25, 2025 is postponed. This guide.
Other Support
“other support” is sometimes referred to as “current and pending support” or “active and pending support.” find instructions, blank format pages,. As the largest public funder of biomedical research in the world, nih supports a variety of programs from grants and contracts to. This guide notice informs the extramural community of nih’s adoption of common forms for biographical sketch and.
“Other Support” Is Sometimes Referred To As “Current And Pending Support” Or “Active And Pending Support.” Find Instructions, Blank Format Pages,.
This guide notice informs the extramural community of nih’s adoption of common forms for biographical sketch and current and. As the largest public funder of biomedical research in the world, nih supports a variety of programs from grants and contracts to. Applicants/recipients will be required to use science experts network curriculum vitae (sciencv) for preparing and certifying nih. The nih's adoption of the common forms (biosketch and current and pending (other) support) originally scheduled for may 25, 2025 is.
Continue To Use The Nih.
The new requirement to train researchers to comply with other support disclosure requirements adds to the current policy (see nih. Nih's adoption of the common form for current and pending (other) support originally scheduled for may 25, 2025 is postponed.