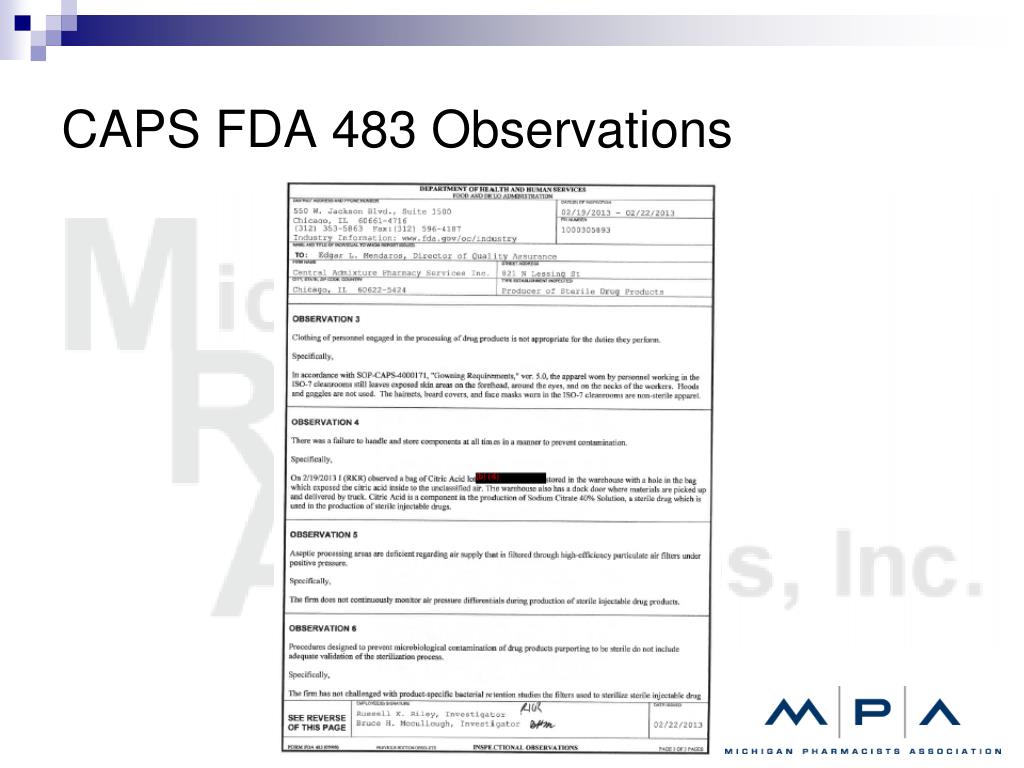
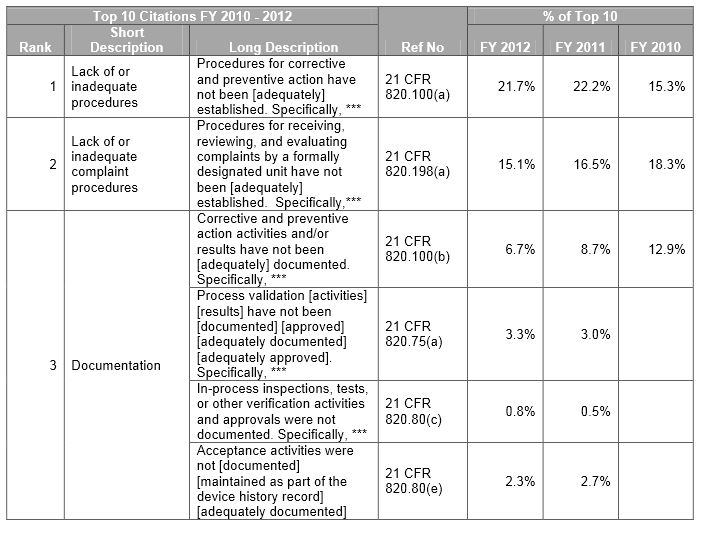
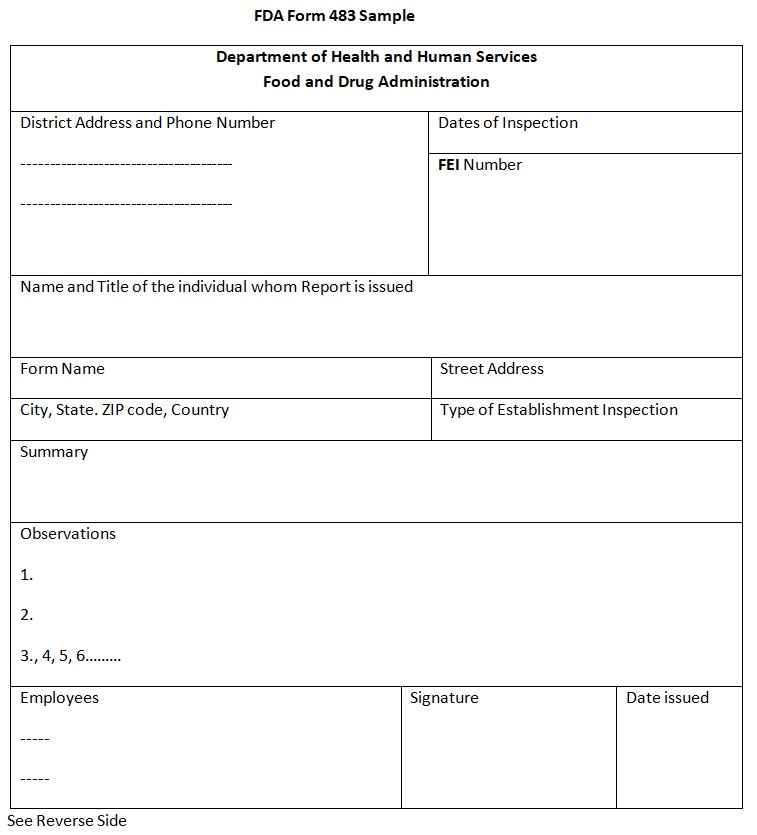
Fda Form 483 Observations - Call the fda consumer complaint coordinator for your state or region. Fda uses science and data to ensure that approved drugs are of a high quality, safe, and effective. Recalls, market withdrawals, & safety alerts the list below provides information gathered from press releases and other public notices about certain. The fda is responsible for protecting the public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological. Learn more about the fda’s role in reviewing, approving,. Fda fulfills this responsibility by ensuring the security of the food supply and by fostering development of medical products to respond to.
Fda uses science and data to ensure that approved drugs are of a high quality, safe, and effective. Fda fulfills this responsibility by ensuring the security of the food supply and by fostering development of medical products to respond to. Recalls, market withdrawals, & safety alerts the list below provides information gathered from press releases and other public notices about certain. The fda is responsible for protecting the public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological. Call the fda consumer complaint coordinator for your state or region. Learn more about the fda’s role in reviewing, approving,.
The fda is responsible for protecting the public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological. Call the fda consumer complaint coordinator for your state or region. Fda uses science and data to ensure that approved drugs are of a high quality, safe, and effective. Recalls, market withdrawals, & safety alerts the list below provides information gathered from press releases and other public notices about certain. Learn more about the fda’s role in reviewing, approving,. Fda fulfills this responsibility by ensuring the security of the food supply and by fostering development of medical products to respond to.
LOGO
Recalls, market withdrawals, & safety alerts the list below provides information gathered from press releases and other public notices about certain. Fda fulfills this responsibility by ensuring the security of the food supply and by fostering development of medical products to respond to. Fda uses science and data to ensure that approved drugs are of a high quality, safe, and.
LOGO
The fda is responsible for protecting the public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological. Call the fda consumer complaint coordinator for your state or region. Fda fulfills this responsibility by ensuring the security of the food supply and by fostering development of medical products to respond to. Fda uses science and data.
Fda 483 Response Template
Fda uses science and data to ensure that approved drugs are of a high quality, safe, and effective. Fda fulfills this responsibility by ensuring the security of the food supply and by fostering development of medical products to respond to. Call the fda consumer complaint coordinator for your state or region. Recalls, market withdrawals, & safety alerts the list below.
PPT Regulatory Issues in Sterile Compounding PowerPoint Presentation
The fda is responsible for protecting the public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological. Call the fda consumer complaint coordinator for your state or region. Recalls, market withdrawals, & safety alerts the list below provides information gathered from press releases and other public notices about certain. Fda fulfills this responsibility by ensuring.
FDA Form 483 Top Ten Observations for Medical Devices SPK and Associates
Recalls, market withdrawals, & safety alerts the list below provides information gathered from press releases and other public notices about certain. Call the fda consumer complaint coordinator for your state or region. Fda fulfills this responsibility by ensuring the security of the food supply and by fostering development of medical products to respond to. Fda uses science and data to.
PPT FDA form 483 observations and warning letters what is the
Call the fda consumer complaint coordinator for your state or region. Fda uses science and data to ensure that approved drugs are of a high quality, safe, and effective. Fda fulfills this responsibility by ensuring the security of the food supply and by fostering development of medical products to respond to. Learn more about the fda’s role in reviewing, approving,..
FDA Form 483 Warning Letters How to Handle, Form, Example » Pharmaguddu
Fda fulfills this responsibility by ensuring the security of the food supply and by fostering development of medical products to respond to. Fda uses science and data to ensure that approved drugs are of a high quality, safe, and effective. Learn more about the fda’s role in reviewing, approving,. Recalls, market withdrawals, & safety alerts the list below provides information.
LOGO
Call the fda consumer complaint coordinator for your state or region. Learn more about the fda’s role in reviewing, approving,. Fda fulfills this responsibility by ensuring the security of the food supply and by fostering development of medical products to respond to. Recalls, market withdrawals, & safety alerts the list below provides information gathered from press releases and other public.
FDA Form 483 Observations and FDA Warning Letters What’s the Difference?
Call the fda consumer complaint coordinator for your state or region. Fda uses science and data to ensure that approved drugs are of a high quality, safe, and effective. Recalls, market withdrawals, & safety alerts the list below provides information gathered from press releases and other public notices about certain. Fda fulfills this responsibility by ensuring the security of the.
483 Inspection Observation Responses Customs & International Trade
Fda fulfills this responsibility by ensuring the security of the food supply and by fostering development of medical products to respond to. Learn more about the fda’s role in reviewing, approving,. Call the fda consumer complaint coordinator for your state or region. The fda is responsible for protecting the public health by ensuring the safety, efficacy, and security of human.
Fda Uses Science And Data To Ensure That Approved Drugs Are Of A High Quality, Safe, And Effective.
Call the fda consumer complaint coordinator for your state or region. The fda is responsible for protecting the public health by ensuring the safety, efficacy, and security of human and veterinary drugs, biological. Fda fulfills this responsibility by ensuring the security of the food supply and by fostering development of medical products to respond to. Learn more about the fda’s role in reviewing, approving,.