Ema Templates - The ema was founded after more than seven years of negotiations among eu governments and replaced the committee for proprietary medicinal products. Parents/guardians and providers can find details about eligible services. The european medicines agency is a decentralised agency of the european union responsible for the evaluation, supervision and safety monitoring of. It is responsible for the scientific evaluation,. The european medicines agency (ema) is a decentralised agency of the european union (eu). Ema welcomes the publication of the european commission’s (ec) new variations guidelines , which streamline the lifecycle. Providers are now able to onboard into the education market assistant (ema).
The european medicines agency (ema) is a decentralised agency of the european union (eu). Providers are now able to onboard into the education market assistant (ema). It is responsible for the scientific evaluation,. Parents/guardians and providers can find details about eligible services. The european medicines agency is a decentralised agency of the european union responsible for the evaluation, supervision and safety monitoring of. The ema was founded after more than seven years of negotiations among eu governments and replaced the committee for proprietary medicinal products. Ema welcomes the publication of the european commission’s (ec) new variations guidelines , which streamline the lifecycle.
It is responsible for the scientific evaluation,. Ema welcomes the publication of the european commission’s (ec) new variations guidelines , which streamline the lifecycle. Parents/guardians and providers can find details about eligible services. The european medicines agency (ema) is a decentralised agency of the european union (eu). The european medicines agency is a decentralised agency of the european union responsible for the evaluation, supervision and safety monitoring of. Providers are now able to onboard into the education market assistant (ema). The ema was founded after more than seven years of negotiations among eu governments and replaced the committee for proprietary medicinal products.
W302 EMA Research Essay Plan Template 23J onwards amended W302 EMA
The european medicines agency (ema) is a decentralised agency of the european union (eu). It is responsible for the scientific evaluation,. Parents/guardians and providers can find details about eligible services. The ema was founded after more than seven years of negotiations among eu governments and replaced the committee for proprietary medicinal products. The european medicines agency is a decentralised agency.
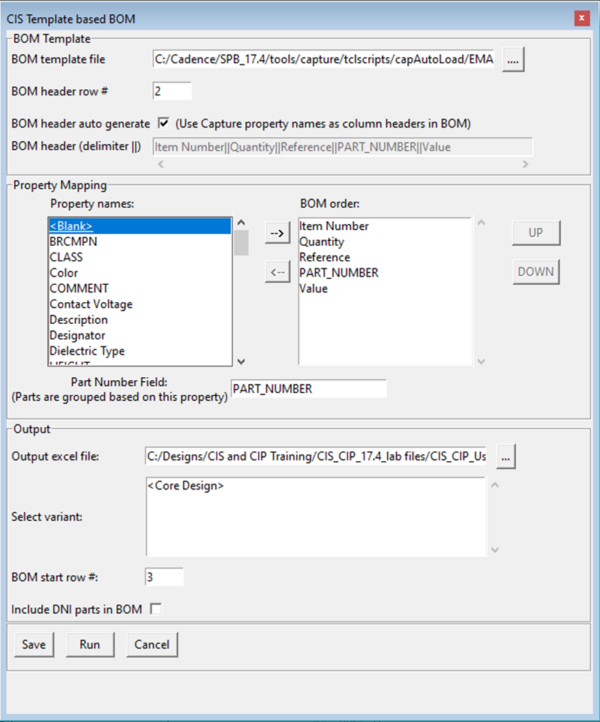
How to Create a BOM with a Template EMA Design Automation
The european medicines agency (ema) is a decentralised agency of the european union (eu). The ema was founded after more than seven years of negotiations among eu governments and replaced the committee for proprietary medicinal products. Parents/guardians and providers can find details about eligible services. The european medicines agency is a decentralised agency of the european union responsible for the.
A translator’s guide to the EMA templates Signs & Symptoms of Translation
The european medicines agency is a decentralised agency of the european union responsible for the evaluation, supervision and safety monitoring of. The ema was founded after more than seven years of negotiations among eu governments and replaced the committee for proprietary medicinal products. It is responsible for the scientific evaluation,. Ema welcomes the publication of the european commission’s (ec) new.
A translator’s guide to the EMA templates Signs & Symptoms of Translation
The european medicines agency (ema) is a decentralised agency of the european union (eu). It is responsible for the scientific evaluation,. The ema was founded after more than seven years of negotiations among eu governments and replaced the committee for proprietary medicinal products. The european medicines agency is a decentralised agency of the european union responsible for the evaluation, supervision.
Tradetron EMA crossover strategy template Nifty Max YouTube
Parents/guardians and providers can find details about eligible services. The european medicines agency (ema) is a decentralised agency of the european union (eu). It is responsible for the scientific evaluation,. Providers are now able to onboard into the education market assistant (ema). Ema welcomes the publication of the european commission’s (ec) new variations guidelines , which streamline the lifecycle.
Grundlagen für medizinische Übersetzer 2 EMATemplates
The european medicines agency (ema) is a decentralised agency of the european union (eu). Parents/guardians and providers can find details about eligible services. It is responsible for the scientific evaluation,. The ema was founded after more than seven years of negotiations among eu governments and replaced the committee for proprietary medicinal products. Providers are now able to onboard into the.
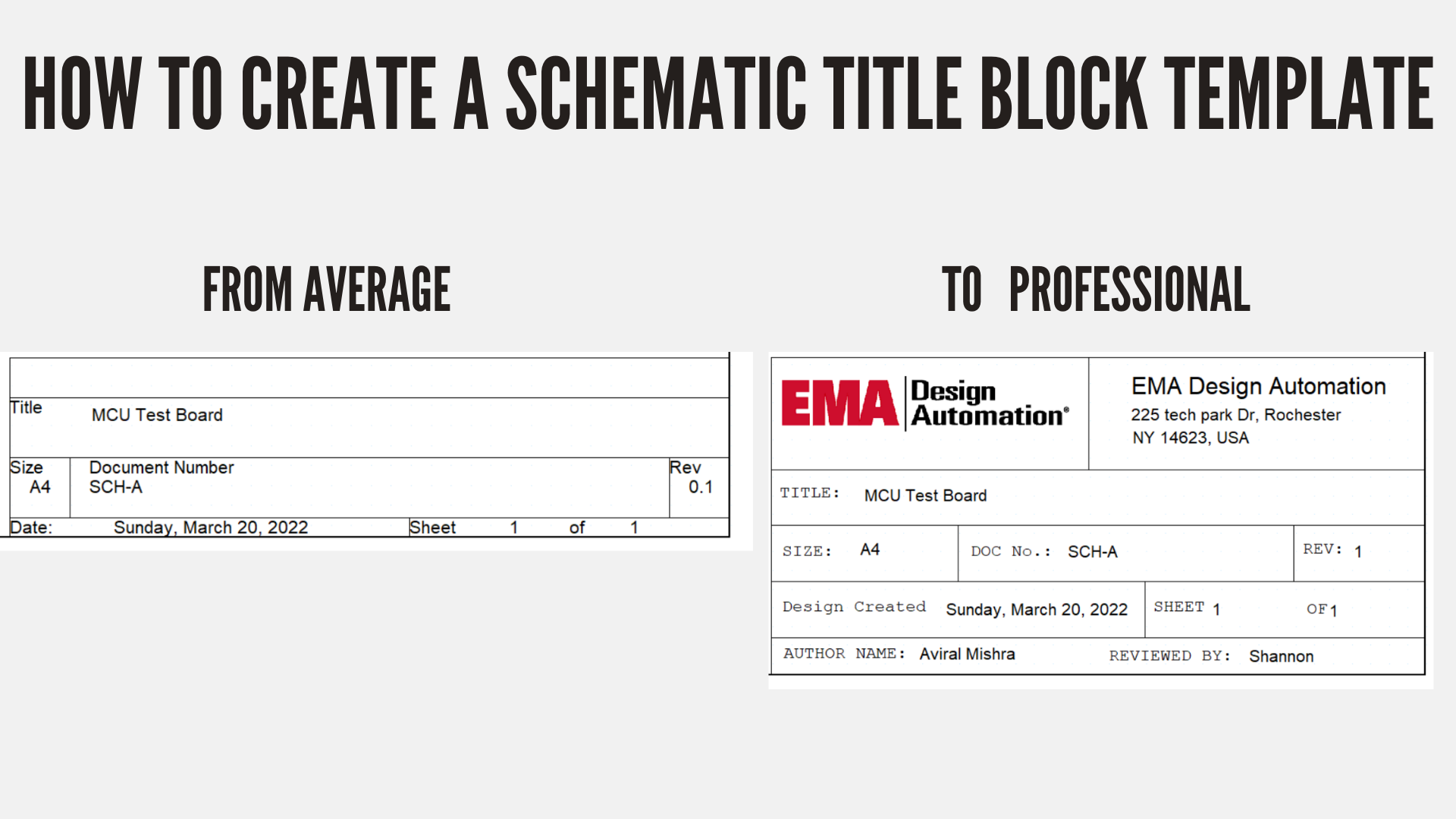
How to Create Schematic Templates EMA Design Automation
Parents/guardians and providers can find details about eligible services. The european medicines agency (ema) is a decentralised agency of the european union (eu). The european medicines agency is a decentralised agency of the european union responsible for the evaluation, supervision and safety monitoring of. The ema was founded after more than seven years of negotiations among eu governments and replaced.
Ema Product Information Templates prntbl.concejomunicipaldechinu.gov.co
It is responsible for the scientific evaluation,. Parents/guardians and providers can find details about eligible services. The european medicines agency is a decentralised agency of the european union responsible for the evaluation, supervision and safety monitoring of. The ema was founded after more than seven years of negotiations among eu governments and replaced the committee for proprietary medicinal products. The.
What changed in the latest EMA QRD template update Mastermind
Providers are now able to onboard into the education market assistant (ema). The ema was founded after more than seven years of negotiations among eu governments and replaced the committee for proprietary medicinal products. It is responsible for the scientific evaluation,. Ema welcomes the publication of the european commission’s (ec) new variations guidelines , which streamline the lifecycle. The european.
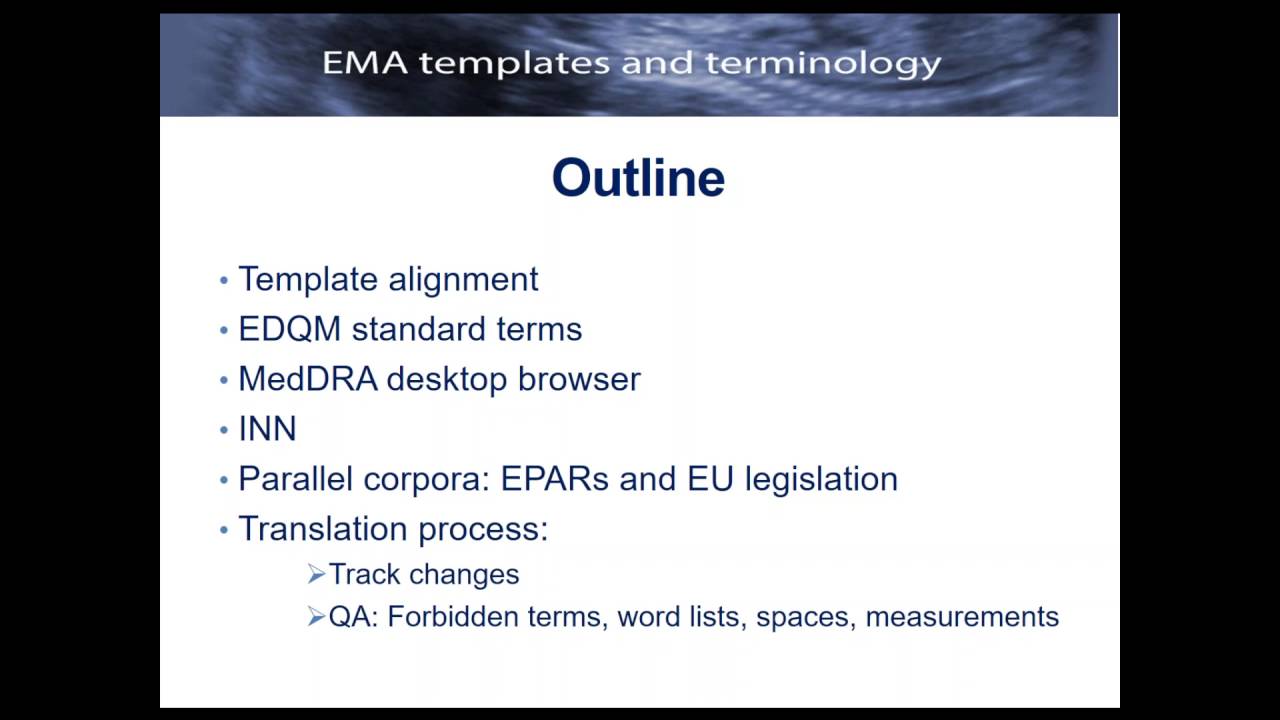
EMA templates and EU terminology for medical translators Beyond the
Ema welcomes the publication of the european commission’s (ec) new variations guidelines , which streamline the lifecycle. The european medicines agency is a decentralised agency of the european union responsible for the evaluation, supervision and safety monitoring of. It is responsible for the scientific evaluation,. Providers are now able to onboard into the education market assistant (ema). The ema was.
The European Medicines Agency (Ema) Is A Decentralised Agency Of The European Union (Eu).
Providers are now able to onboard into the education market assistant (ema). The ema was founded after more than seven years of negotiations among eu governments and replaced the committee for proprietary medicinal products. It is responsible for the scientific evaluation,. Ema welcomes the publication of the european commission’s (ec) new variations guidelines , which streamline the lifecycle.
Parents/Guardians And Providers Can Find Details About Eligible Services.
The european medicines agency is a decentralised agency of the european union responsible for the evaluation, supervision and safety monitoring of.