Debriefing Form - The form should not only include an explanation of the deception in the current study, but also a brief explanation about why deception must take place. This form provides background about our research to help you learn more about why we are doing this study. To debrief participants at the end of a study is mandatory in case of deception and highly recommended in all the others cases. This study [explain and describe the purpose of the study (hypothesis and description of the study]. [if there is deception, expl ich you. Please feel free to ask any questions or to. Include, if applicable, and provide a list of medical/mental health providers for the. Copy “right to withdraw” information from the original consent.
This study [explain and describe the purpose of the study (hypothesis and description of the study]. To debrief participants at the end of a study is mandatory in case of deception and highly recommended in all the others cases. The form should not only include an explanation of the deception in the current study, but also a brief explanation about why deception must take place. [if there is deception, expl ich you. Please feel free to ask any questions or to. Copy “right to withdraw” information from the original consent. Include, if applicable, and provide a list of medical/mental health providers for the. This form provides background about our research to help you learn more about why we are doing this study.
This form provides background about our research to help you learn more about why we are doing this study. Please feel free to ask any questions or to. The form should not only include an explanation of the deception in the current study, but also a brief explanation about why deception must take place. Include, if applicable, and provide a list of medical/mental health providers for the. This study [explain and describe the purpose of the study (hypothesis and description of the study]. [if there is deception, expl ich you. To debrief participants at the end of a study is mandatory in case of deception and highly recommended in all the others cases. Copy “right to withdraw” information from the original consent.
Obstetric Team Debriefing Form Safe Motherhood Initiative PDF
Copy “right to withdraw” information from the original consent. The form should not only include an explanation of the deception in the current study, but also a brief explanation about why deception must take place. To debrief participants at the end of a study is mandatory in case of deception and highly recommended in all the others cases. [if there.
debriefing Doc Template pdfFiller
To debrief participants at the end of a study is mandatory in case of deception and highly recommended in all the others cases. Include, if applicable, and provide a list of medical/mental health providers for the. This form provides background about our research to help you learn more about why we are doing this study. Please feel free to ask.
Top 12 Debriefing Form Templates free to download in PDF format
The form should not only include an explanation of the deception in the current study, but also a brief explanation about why deception must take place. Please feel free to ask any questions or to. [if there is deception, expl ich you. This study [explain and describe the purpose of the study (hypothesis and description of the study]. Include, if.
Top 12 Debriefing Form Templates free to download in PDF format
To debrief participants at the end of a study is mandatory in case of deception and highly recommended in all the others cases. This form provides background about our research to help you learn more about why we are doing this study. [if there is deception, expl ich you. The form should not only include an explanation of the deception.
Debriefing Checklist Best Self Help Solutions
This study [explain and describe the purpose of the study (hypothesis and description of the study]. [if there is deception, expl ich you. Please feel free to ask any questions or to. Copy “right to withdraw” information from the original consent. The form should not only include an explanation of the deception in the current study, but also a brief.
Nur166 debriefing w2 debrifing form week 2 Due April 29thp
The form should not only include an explanation of the deception in the current study, but also a brief explanation about why deception must take place. Copy “right to withdraw” information from the original consent. This study [explain and describe the purpose of the study (hypothesis and description of the study]. Include, if applicable, and provide a list of medical/mental.
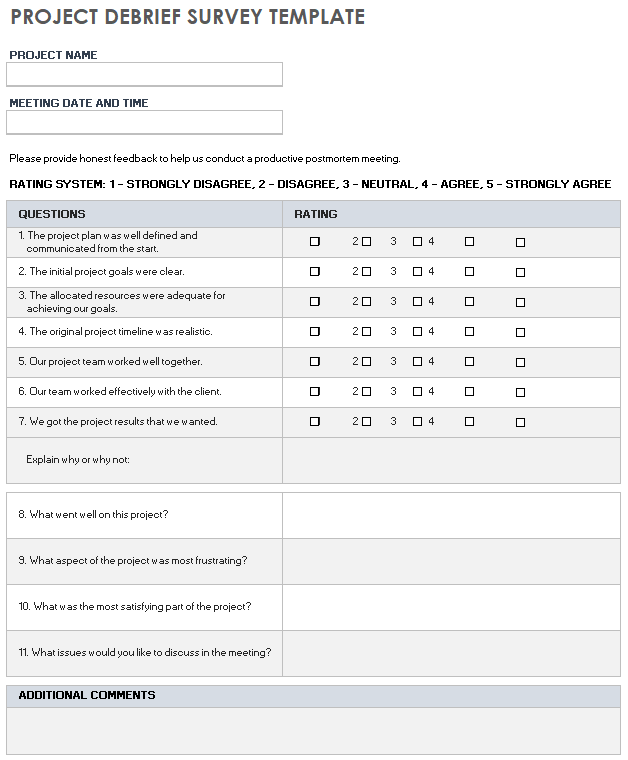
Free Project Debrief Templates Smartsheet
To debrief participants at the end of a study is mandatory in case of deception and highly recommended in all the others cases. Include, if applicable, and provide a list of medical/mental health providers for the. Copy “right to withdraw” information from the original consent. This form provides background about our research to help you learn more about why we.
debriefing — Blog — NUEM Blog
The form should not only include an explanation of the deception in the current study, but also a brief explanation about why deception must take place. This study [explain and describe the purpose of the study (hypothesis and description of the study]. This form provides background about our research to help you learn more about why we are doing this.
PPT D ebriefing PowerPoint Presentation, free download ID242092
Please feel free to ask any questions or to. This form provides background about our research to help you learn more about why we are doing this study. Copy “right to withdraw” information from the original consent. The form should not only include an explanation of the deception in the current study, but also a brief explanation about why deception.
10 Debriefing Form Templates free to download in PDF
To debrief participants at the end of a study is mandatory in case of deception and highly recommended in all the others cases. Please feel free to ask any questions or to. Include, if applicable, and provide a list of medical/mental health providers for the. [if there is deception, expl ich you. The form should not only include an explanation.
Copy “Right To Withdraw” Information From The Original Consent.
The form should not only include an explanation of the deception in the current study, but also a brief explanation about why deception must take place. [if there is deception, expl ich you. Include, if applicable, and provide a list of medical/mental health providers for the. To debrief participants at the end of a study is mandatory in case of deception and highly recommended in all the others cases.
Please Feel Free To Ask Any Questions Or To.
This study [explain and describe the purpose of the study (hypothesis and description of the study]. This form provides background about our research to help you learn more about why we are doing this study.