Ctd Dossier Templates Regulatory Submissions - This article provides a deep dive into regulatory submission strategies, dossier formats (ctd and ectd), best practices for compilation. Dossier templates are structured frameworks that guide pharmaceutical companies in preparing regulatory submissions such as ctd.
Dossier templates are structured frameworks that guide pharmaceutical companies in preparing regulatory submissions such as ctd. This article provides a deep dive into regulatory submission strategies, dossier formats (ctd and ectd), best practices for compilation.
This article provides a deep dive into regulatory submission strategies, dossier formats (ctd and ectd), best practices for compilation. Dossier templates are structured frameworks that guide pharmaceutical companies in preparing regulatory submissions such as ctd.
Regulatory dossier preparation and submission as per CTD format PPTX
This article provides a deep dive into regulatory submission strategies, dossier formats (ctd and ectd), best practices for compilation. Dossier templates are structured frameworks that guide pharmaceutical companies in preparing regulatory submissions such as ctd.
Chapter 15 CTD Format & Regulatory Dossier Preparation
This article provides a deep dive into regulatory submission strategies, dossier formats (ctd and ectd), best practices for compilation. Dossier templates are structured frameworks that guide pharmaceutical companies in preparing regulatory submissions such as ctd.
Structure of Dossier of Medicinal Product Q part ppt video online
This article provides a deep dive into regulatory submission strategies, dossier formats (ctd and ectd), best practices for compilation. Dossier templates are structured frameworks that guide pharmaceutical companies in preparing regulatory submissions such as ctd.
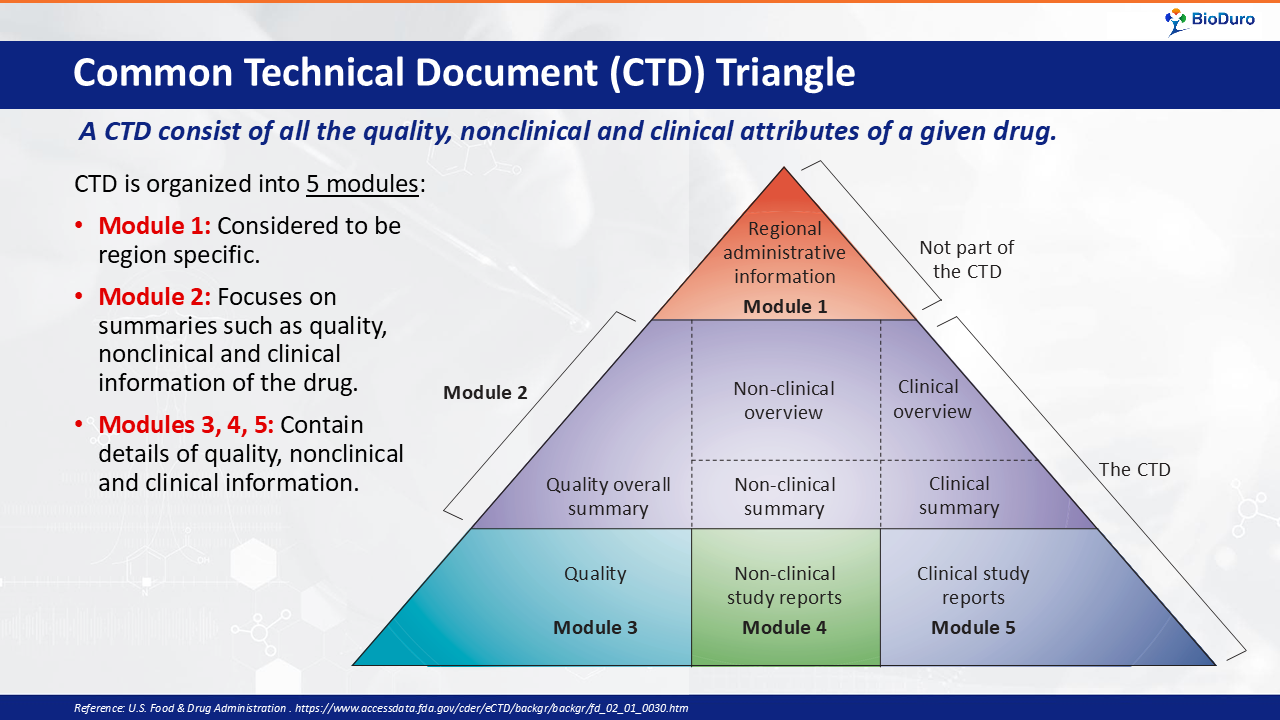
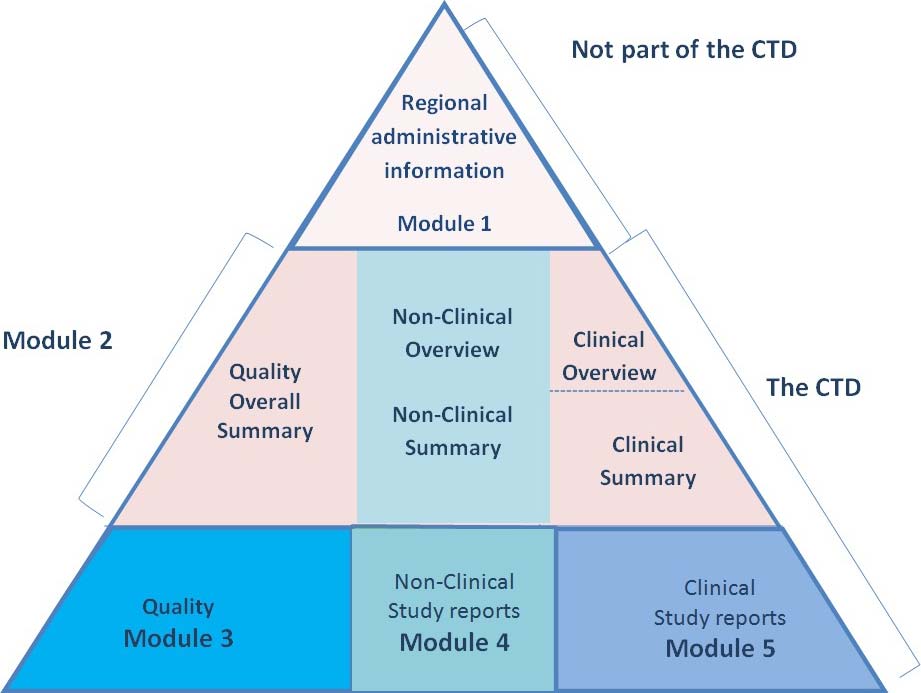
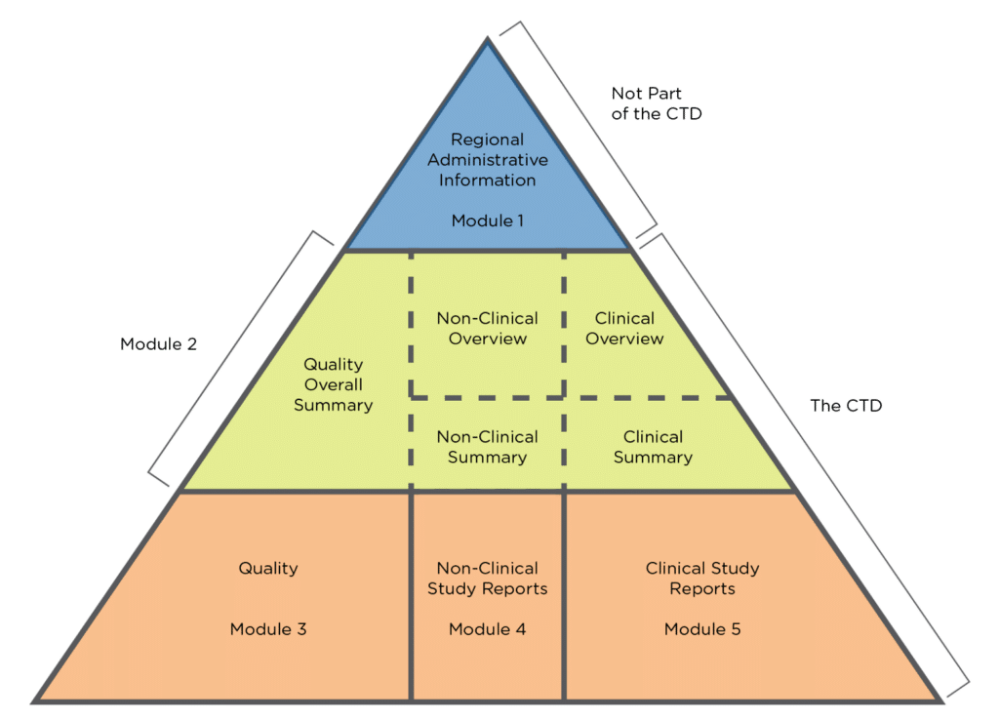
Common Technical Document (CTD) Standard Format for Presenting Data in
This article provides a deep dive into regulatory submission strategies, dossier formats (ctd and ectd), best practices for compilation. Dossier templates are structured frameworks that guide pharmaceutical companies in preparing regulatory submissions such as ctd.
Regulatory dossier preparation and submission as per CTD format PPTX
This article provides a deep dive into regulatory submission strategies, dossier formats (ctd and ectd), best practices for compilation. Dossier templates are structured frameworks that guide pharmaceutical companies in preparing regulatory submissions such as ctd.
(PDF) CTD Dossier Preparation PHARMACEUTICALS
This article provides a deep dive into regulatory submission strategies, dossier formats (ctd and ectd), best practices for compilation. Dossier templates are structured frameworks that guide pharmaceutical companies in preparing regulatory submissions such as ctd.
Skyward Regulatory Professional on LinkedIn regulatoryaffairs ctd
This article provides a deep dive into regulatory submission strategies, dossier formats (ctd and ectd), best practices for compilation. Dossier templates are structured frameworks that guide pharmaceutical companies in preparing regulatory submissions such as ctd.
Ts Diagram Obtained For All Ctd Cruises Charac Teristic
Dossier templates are structured frameworks that guide pharmaceutical companies in preparing regulatory submissions such as ctd. This article provides a deep dive into regulatory submission strategies, dossier formats (ctd and ectd), best practices for compilation.
Regulatory dossier preparation and submission as per CTD format PPTX
This article provides a deep dive into regulatory submission strategies, dossier formats (ctd and ectd), best practices for compilation. Dossier templates are structured frameworks that guide pharmaceutical companies in preparing regulatory submissions such as ctd.
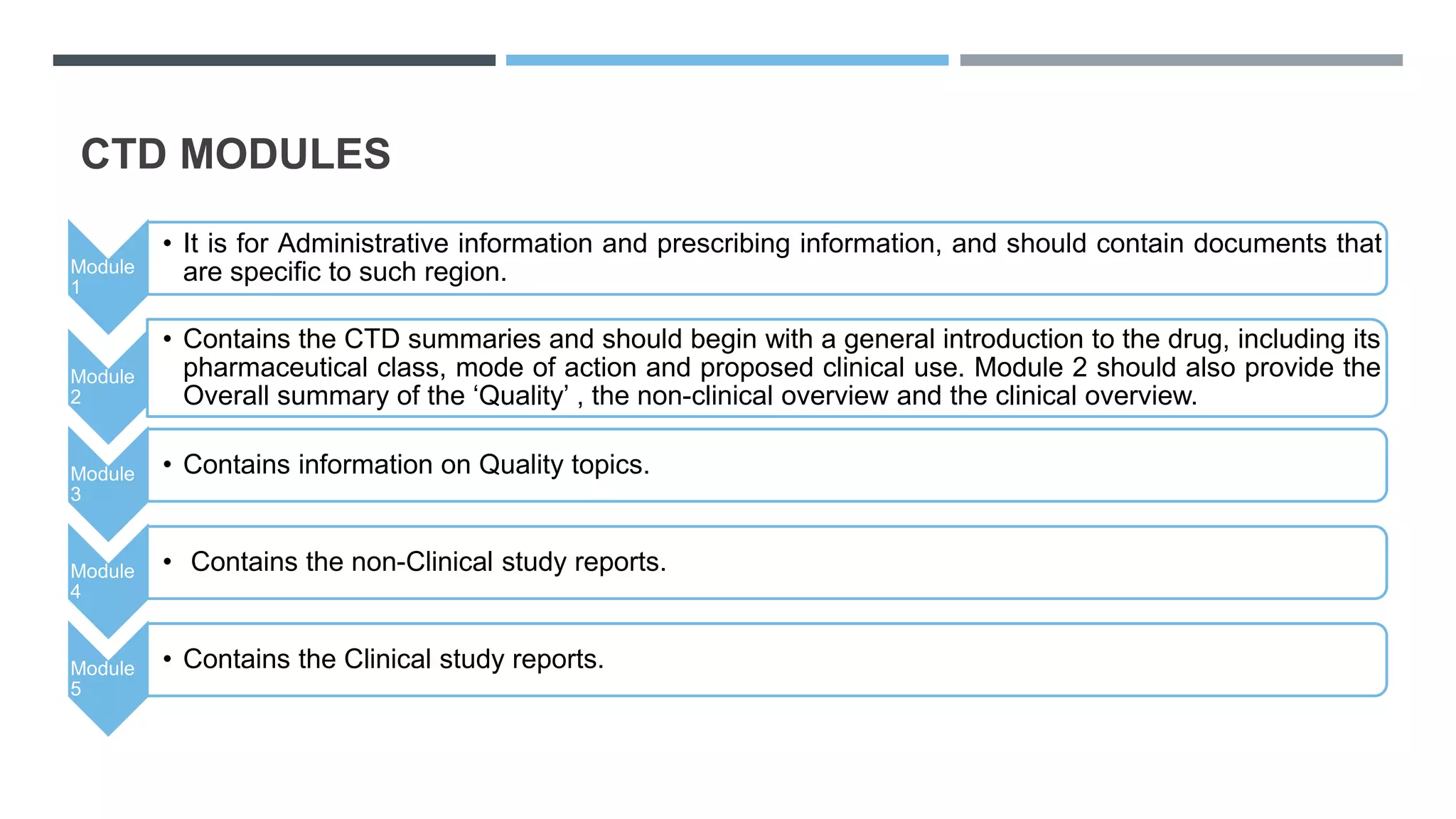
"Understanding the Five Modules of the CTD Format in Regulatory Affairs
Dossier templates are structured frameworks that guide pharmaceutical companies in preparing regulatory submissions such as ctd. This article provides a deep dive into regulatory submission strategies, dossier formats (ctd and ectd), best practices for compilation.
Dossier Templates Are Structured Frameworks That Guide Pharmaceutical Companies In Preparing Regulatory Submissions Such As Ctd.
This article provides a deep dive into regulatory submission strategies, dossier formats (ctd and ectd), best practices for compilation.


.jpg)